Mikron Tool announce the release of the 3rd edition of the digital Tool Book 2024 - 2027. Today the range of catalog tools includes more than 5’800 single items. These are included in the main catalog together with many additional and useful information for the end user. Divided in machining groups like drilling, milling, or deburring each product range has its exact description with its features and advantages. Additional indications on size, availability, cutting parameters and machining (...)
The Eurotec Newsletter
Machining
SORALUCE INTRODUCES DYNAMIC LINE: STEP INTO PRECISION & DYNAMICS WITH THE NEW SORALUCE HIGH RAIL GANTRY MACHINES

Soraluce, a trailblazer in milling, boring, multitasking and automated solutions announces the launch of its groundbreaking Dynamic Line, featuring the New High Rail Gantry Machines. These innovative machines epitomize precision, dynamics, and efficiency, poised to revolutionize manufacturing processes across diverse industries. The High Rail Gantry Machines from Soraluce are engineered to propel manufacturing operations to unprecedented levels of performance. Offering high-speed cutting (...)
Automation
EMERSON TO SHOWCASE FLOOR TO CLOUD™ FACTORY AUTOMATION SOLUTIONS AT HANNOVER MESSE 2024 (HALL 11, BOOTH C20)


Global technology, software and engineering leader, Emerson will present the future of automation through its Floor to Cloud™ approach and innovative automation solutions at Hannover Messe in Hannover, Germany on April 22-26, 2024. Emerson will demonstrate how real-time visibility and control can drive sustainability, enhance overall equipment effectiveness (OEE) and empower teams using data-informed decision-making. This is the first year Emerson’s booth will be housed in hall 11 at booth (...)
Trade show
365 days of networking in the medical technology industry - Launch of the new MedtecLIVE Community

At the beginning of February, MedtecLIVE, the leading European trade fair for the production of medical technology products and door opener to the important European consumer and production market, launched a new format: the digital MedtecLIVE Community. "Following the relaunch of the website, the community now offers the unique opportunity to continue the trade fair experience digitally throughout the year. We connect exhibitors and visitors with each other and with many other players in (...)
Electronics
BINDER FOR EFFICIENT, AUTOMATED PROCESSES


Several M12 connector series from binder are available in versions for surface mount technology (SMT). On the one hand, they are advantageous for integration into space-critical designs, on the other hand, the good automation capability in further processing offers enormous savings potential. binder, a leading supplier of industrial circular connectors, offers products from several M12 series as surface mount devices (SMDs). They are suitable for further processing in fully automated (...)
Motion
Kollmorgen erweitert die Einsatzmöglichkeiten des AKD2G mit der Einführung neuer synchronisierter Kommunikationsprotokolle

Der aktualisierte Kollmorgen AKD2G Servoantrieb verfügt über neue Kommunikationsprotokolle, die eine einfachere Integration mit Steuerungen von Drittanbietern ermöglichen und gleichzeitig eine branchenführende Leistung bieten. Kollmorgen, ein weltweit führender Anbieter von Antriebssystemen, hat heute das neueste Update seines Servoreglers AKD2G angekündigt. Mit der Einführung neuer Funktionen erweitert Kollmorgen sein Angebot und unterstützt nun neben den zeitlich synchronisierten (...)
Motion
Kollmorgen expands AKD2G versatility with the launch of new synchronized communication protocols

The updated Kollmorgen AKD2G servo drive features new communication protocols that support easier integration with third-party controllers while delivering industry-leading performance. Kollmorgen, a global leader in motion control systems, today announced the latest update to its AKD2G servo drive. With the introduction of these new features, Kollmorgen has broadened its offerings to additionally support PROFINET IRT and Ethernet/IP with CIP Sync alongside CANopen®*, EtherCAT®*, and FSoE (...)
Tooling
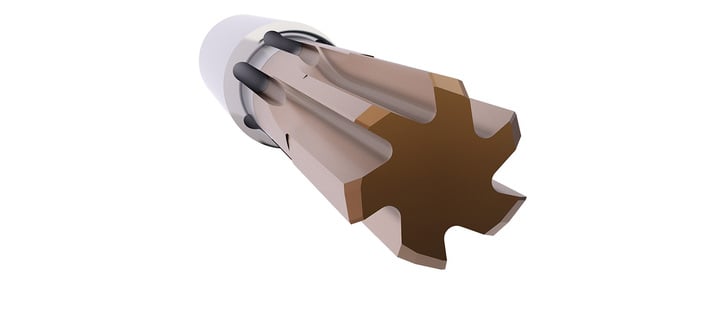
HORN HAS DEVELOPED THE NEW SG66 GRADE FOR TURNING WORKPIECES HAVING DIFFERENT HARDNESS ZONES


When machining turned parts with hardened surface layers or with an interrupted cut, users quickly reach the limits of CBN inserts. This is where the new grade comes in. In combination with the fine-grain carbide, the aluminium-titanium-silicon chromium nitride layer delivers high performance when machining hardened steels up to 58 HRC. The maximum allowable temperature is 1,200 degrees Celsius (2,192 degrees Fahrenheit). Due to the high flexural strength of the carbide substrate, (...)
Tooling
Seco Nanojet Reamers optimize chip control to eliminate scrapped parts

Seco Nanojet Solid Carbide Reamers enhance chip control with an innovative through-coolant outlet for optimal chip evacuation. This design eliminates costly scrapped parts, jamming and edge damage to increase safety, part quality and tool life. Better coolant flow for better parts Critical reaming operations require stable, secure, predictable tools. On blind and through bores, Seco Nanojet Solid Carbide Reamers extend the proven performance of Seco Nanofix products with innovative (...)
Automation
SCHUNK: DIGITAL AND AUTOMATED HEALTHY PRODUCTION

Schunk presents automated solutions at the Hannover Messe that pave the way for this transformation. "Automation is the key for creating a healthy, efficient and responsible industry," emphasizes Timo Gessmann, Chief Technology Officer of Schunk. Therefore, the technology company is focusing on two main topics this year: "The healthy factory" and "Innovation through Collaboration." The aim is to design a production process that is healthy and economically successful for people and the (...)